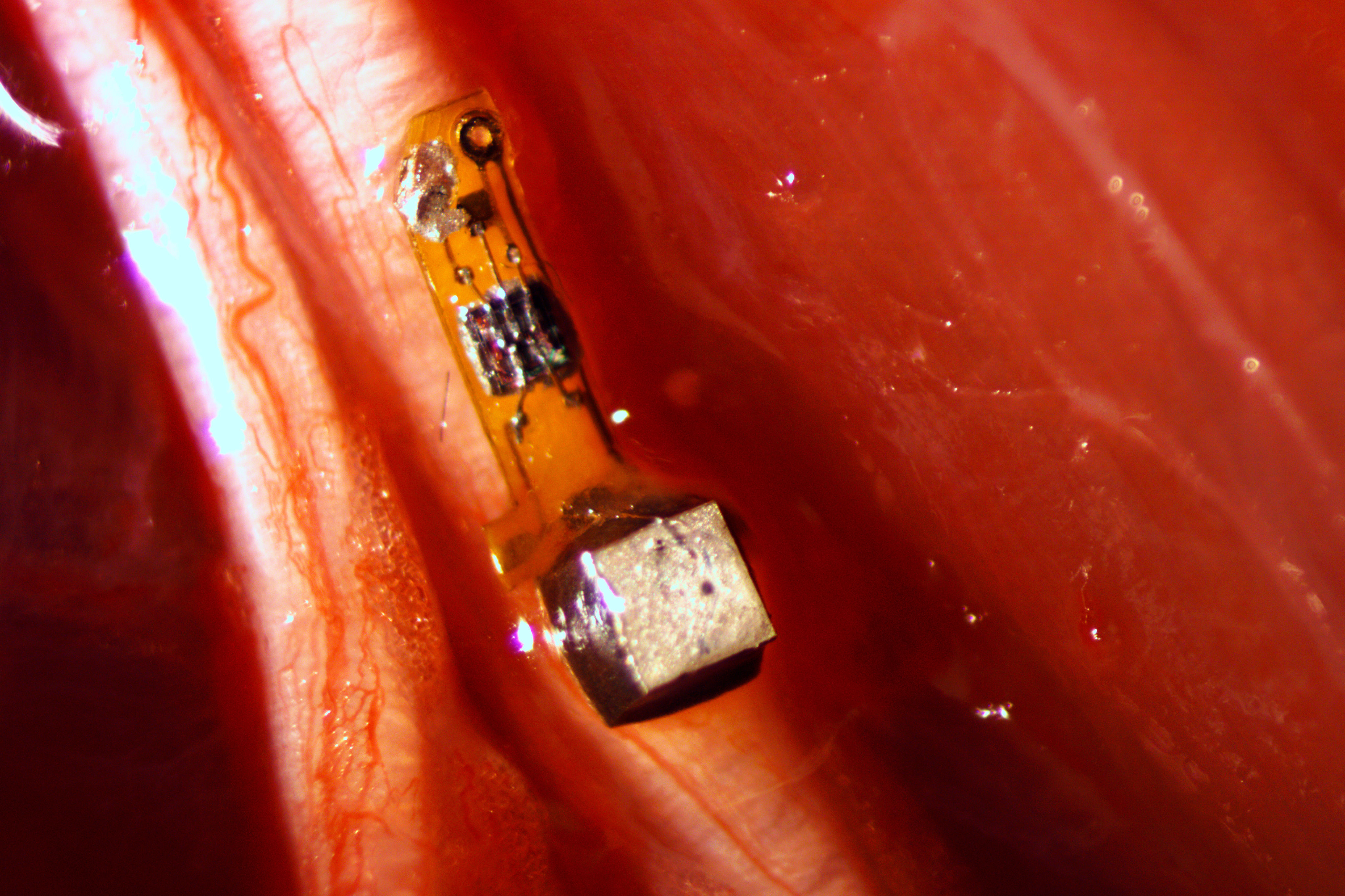
University of California, Berkeley engineers have built the first dust-sized, wireless sensors that can be implanted in the body, bringing closer the day when a Fitbit-like device could monitor internal nerves, muscles or organs in real time.
Because these batteryless sensors could also be used to stimulate nerves and muscles, the technology also opens the door to “electroceuticals” to treat disorders such as epilepsy or to stimulate the immune system or tamp down inflammation.
READ MORE ON UC BERKELEY | NEWS
Ref: Wireless Recording in the Peripheral Nervous System with Ultrasonic Neural Dust. Neuron (3 August 2016) | DOI: 10.1016/j.neuron.2016.06.034 (Open Access)
ABSTRACT
The emerging field of bioelectronic medicine seeks methods for deciphering and modulating electrophysiological activity in the body to attain therapeutic effects at target organs. Current approaches to interfacing with peripheral nerves and muscles rely heavily on wires, creating problems for chronic use, while emerging wireless approaches lack the size scalability necessary to interrogate small-diameter nerves. Furthermore, conventional electrode-based technologies lack the capability to record from nerves with high spatial resolution or to record independently from many discrete sites within a nerve bundle. Here, we demonstrate neural dust, a wireless and scalable ultrasonic backscatter system for powering and communicating with implanted bioelectronics. We show that ultrasound is effective at delivering power to mm-scale devices in tissue; likewise, passive, battery-less communication using backscatter enables high-fidelity transmission of electromyogram (EMG) and electroneurogram (ENG) signals from anesthetized rats. These results highlight the potential for an ultrasound-based neural interface system for advancing future bioelectronics-based therapies.